Mylan Initiates Voluntary Nationwide Recall of 15 Lots of Valsartan Tablets, USP, Amlodipine and Valsartan Tablets, USP, and Valsartan and Hydrochlorothiazide Tablets, USP, Due to the Detection of Trace Amounts of NDEA (N-Nitrosodiethylamine) Impurity Fou
The finished products are manufactured by
|
NDC |
Product Description |
Strength |
Size |
Lot Number |
Expiry |
|
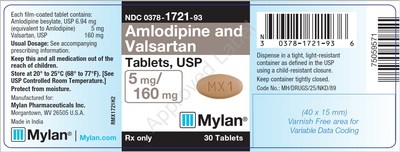
0378-1721-93 |
Amlodipine and Valsartan Tablets, USP |
5mg/160mg |
Bottles of 30 |
3066051 |
3/2019 |
|
0378-1722-93 |
Amlodipine and Valsartan Tablets, USP |
10mg/160mg |
Bottles of 30 |
3079500 |
1/2020 |
|
0378-1724-93 |
Amlodipine and Valsartan Tablets, USP |
10mg/320mg |
Bottles of 30 |
3061986 |
11/2018 |
|
0378-1724-93 |
Amlodipine and Valsartan Tablets, USP |
10mg/320mg |
Bottles of 30 |
3079709 |
1/2020 |
|
0378-1724-93 |
Amlodipine and Valsartan Tablets, USP |
10mg/320mg |
Bottles of 30 |
3077618 |
11/2019 |
|
0378-1724-93 |
Amlodipine and Valsartan Tablets, USP |
10mg/320mg |
Bottles of 30 |
3079708 |
1/2020 |
|
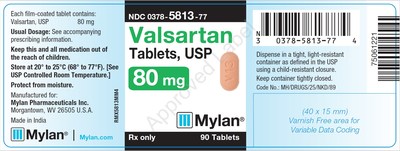
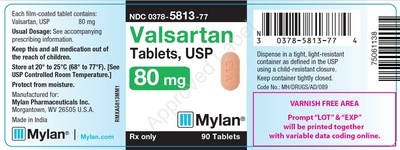
0378-5813-77 |
Valsartan Tablets, USP |
80mg |
Bottles of 90 |
3063782 |
1/2019 |
|
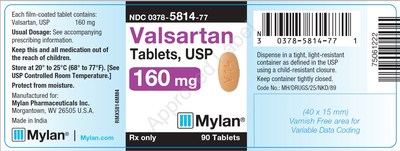
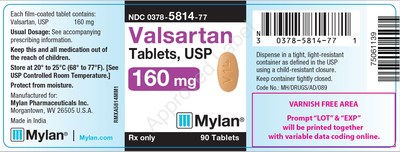
0378-5814-77 |
Valsartan Tablets, USP |
160mg |
Bottles of 90 |
3071352 |
7/2019 |
|
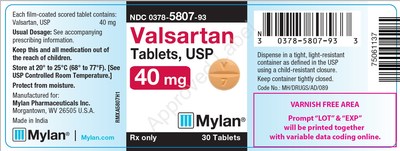
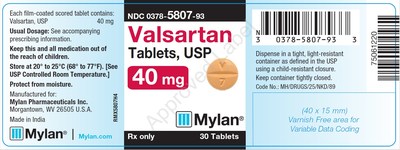
0378-5807-93 |
Valsartan Tablets, USP |
40mg |
Bottles of 30 |
3061169 |
11/2018 |
|
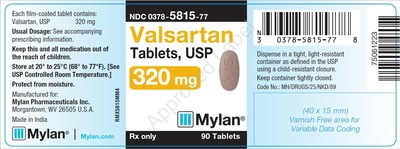
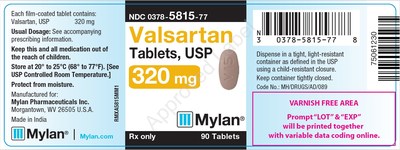
0378-5815-77 |
Valsartan Tablets, USP |
320mg |
Bottles of 90 |
3081499 |
3/2020 |
|
0378-5815-77 |
Valsartan Tablets, USP |
320mg |
Bottles of 90 |
3080009 |
2/2020 |
|
0378-5815-77 |
Valsartan Tablets, USP |
320mg |
Bottles of 90 |
3080010 |
2/2020 |
|
0378-5815-77 |
Valsartan Tablets, USP |
320mg |
Bottles of 90 |
3079205 |
1/2020 |
|
0378-6325-05 |
Valsartan and Hydrochlorothiazide Tablets, USP |
320mg/25mg |
Bottles of 500 |
3084886 |
2/2019 |
|
0378-6325-05 |
Valsartan and Hydrochlorothiazide Tablets, USP |
320mg/25mg |
Bottles of 500 |
3093804 |
12/2019 |
Valsartan is used for the treatment of high blood pressure for the treatment of heart failure, and to reduce cardiovascular mortality following myocardial infarction. Valsartan in combination with amlodipine or hydrochlorothiazide is used for the treatment of high blood pressure. Patients should contact their pharmacist or physician who can advise them about an alternative treatment prior to returning their medication. Patients who are on valsartan should continue taking their medication, as the risk of harm to the patient's health may be higher if the treatment is stopped immediately without any alternative treatment.
Mylan is notifying its distributors and customers by letter and is arranging for return of all recalled products. Wholesalers, retailers and consumers that are in possession of recalled product should contact
Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to using these drug products.
- Adverse reactions or quality problems experienced with the use of this product may be reported to the
FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax. Complete and submit the report Online: www.fda.gov/medwatch/report.htm - Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form or submit by fax to 1-800-
FDA -0178.
This recall is being conducted with the knowledge of the
About Mylan
Mylan is a global pharmaceutical company committed to setting new standards in healthcare. Working together around the world to provide 7 billion people access to high quality medicine, we innovate to satisfy unmet needs; make reliability and service excellence a habit; do what's right, not what's easy; and impact the future through passionate global leadership. We offer a growing portfolio of more than 7,500 marketed products around the world, including antiretroviral therapies on which more than 40% of people being treated for HIV/AIDS globally depend. We market our products in more than 165 countries and territories. We are one of the world's largest producers of active pharmaceutical ingredients. Every member of our approximately 35,000-strong workforce is dedicated to creating better health for a better world, one person at a time. Learn more at Mylan.com. We routinely post information that may be important to investors on our website at investor.mylan.com.
Product Photos



![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/mylan-initiates-voluntary-nationwide-recall-of-15-lots-of-valsartan-tablets-usp-amlodipine-and-valsartan-tablets-usp-and-valsartan-and-hydrochlorothiazide-tablets-usp-due-to-the-detection-of-trace-amounts-of-ndea-n-nitrosod-300753937.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/mylan-initiates-voluntary-nationwide-recall-of-15-lots-of-valsartan-tablets-usp-amlodipine-and-valsartan-tablets-usp-and-valsartan-and-hydrochlorothiazide-tablets-usp-due-to-the-detection-of-trace-amounts-of-ndea-n-nitrosod-300753937.html
SOURCE
Christine Waller (Media) 724.514.1968; Melissa Trombetta (Investors) 724.514.1813
